The dawn of the chemical evolution of the Universe
How did the simple mixture of hydrogen and helium produced in the first three minutes after the Big Bang evolve in the chemically rich and complex universe we live in today? When and how did the first molecules appear in the hostile environment of the rapidly evolving and expanding Universe? The study of primordial chemistry aims exactly at answering these questions. In the last forty years, chemical networks of increasing complexity have been developed to follow the changing composition of the baryonic component of the Universe in the half billion years that preceded the formation of the first stars that manufactured the heavy elements of which we are made of. Nothing could have happened if the universe had not gone through the epoch in which the ionized hydrogen recombined to form neutral hydrogen, leaving behind a tiny but crucial fraction of free electrons at the so-called "recombination epoch", four hundred thousand years after the Big Bang. These electrons acted as catalysts for the occurrence of gas-phase reactions, mainly producing neutral molecules (molecular hydrogen, the simplest molecule containing the most abundant element, and its deuterated isotopologue), and various anions and cations from helium and the trace elements deuterium and lithium.
This story is the subject of a review paper that appeared in early 2013 in the Annual Reviews of Astronomy and Astrophysics, authored by Daniele Galli and Francesco Palla of the INAF-Arcetri Astrophysical Observatory. The need to review the chemical evolution of the Universe stems from the realization that cosmology has fully entered the precision era, thanks to a suite of telescopes such as HST, BOOMERANG, WMAP, and Planck. Today, the reference cosmological model is known with unprecedented accuracy, leaving to the chemistry the remaining uncertainties, whether experimental or theoretical.
The various phases that marked the production of the most interesting species as a function of redshift are shown in the enclosed figure starting from the recombination era (z=2000) to the epoch of first structure formation (z=10). These simple molecules played a fundamental role in allowing the primordial gas to cool and fragment within the dark matter dominated halos, thus producing the first objects that lit up the dark ages. The nature of these first stars, or even massive black holes, is still controversial, but the action of the molecular species thus formed cannot be neglected. Of equal importance is the interaction of the primordial molecules with the photons of the cosmic microwave background that left specific signatures in its spectrum. For some species these calculations require ad-hoc experiments to determine the accurate frequencies of the relevant molecular transitions. For example, the most accurate frequencies for the ro-vibrational transitions of lithium hydrides were obtained in a joint effort at the European Laboratory for Non-Linear Spectroscopy, then at the Arcetri hill.
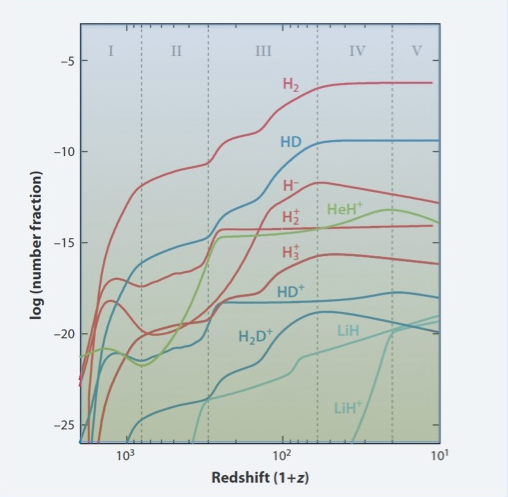
Figure caption: Fractional abundances (relative to the total number of baryons) of the main molecules and ions formed in the early Universe as function of redshift. The vertical dotted lines indicate the boundaries of the five main evolutionary phases that characterize the beginning of the chemical evolution of the Universe.



